Similar documents
Review of the psychological and pedagogical foundations of using a chemical experiment in the study of a school chemistry course. Analysis of the cognitive significance of a chemical experiment in studying the properties of inorganic and organic compounds in a school chemistry course.
certification work, added 10/31/2017
Design of a modern chemistry lesson, its tasks, goals and methods. Features of forms of organization of cognitive activity. Stages of a teaching session in different types of lessons. The personality of the teacher, his role. Using physical instruments in chemistry lessons.
abstract, added 10/17/2010
Integration of chemical content with material from the humanities. Chemical experiment in humanities and socio-economic classes. Using calculation problems in chemistry. Ways to integrate the content of school courses in chemistry and economics.
course work, added 02/18/2011
Possibilities of using information and communication technologies in chemistry lessons. ICT as a means of visibility, as well as a didactic tool (a means of organizing front-line work and control). ICT as a tool for a unified educational environment.
certification work, added 11/17/2016
The use of visual aids in combination with the verbal method in the process of studying chemistry. Participation of students in conducting classes: performing experimental experiments using instruments and reagents. Requirements for didactic teaching aids.
article, added 03/10/2018
Methods of teaching the basics of chemistry at school. Laboratory experiments and practical exercises as part of a student experiment. Organization of a chemical experiment. Study of chemical processes, their description in the form of examples. Samples of work execution.
tutorial, added 10/06/2015
Fundamentals of teaching chemistry, the use of problem-based teaching methods: the formation of chemical language; experiment, its role in the development of schoolchildren’s thinking. Methodology for studying the interaction of metals with salt solutions; experiment on colloidal solutions.
thesis, added 11/08/2010
Theoretical foundations of safety regulations. Simple laboratory equipment. Chemical glassware for special purposes. Ensuring safety and conducting student experiments. Safety rules when working in a chemical laboratory.
course work, added 10/17/2010
Chemical experiment as a specific teaching method. Concept, methodology for organizing and conducting demonstration, student experiments and laboratory workshops. An example of experiments carried out in a laboratory workshop on the topic "Halogens".
test, added 03/22/2014
The essence and significance of a school chemical experiment in modern conditions. Analysis of directions of its development. An example of practical research activities based on a general experiment of an environmental focus for high school.
Verbal-visual teaching methods determine the use of various visual aids in the educational process in combination with the teacher’s word. They are directly related to learning tools and depend on them. In turn, teaching methods impose certain requirements on didactic means. The process of eliminating this contradiction lies at the heart of improving these systems.
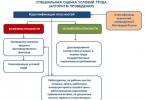
The system of verbal-visual teaching methods and its place in the educational process can be imagined in the form of a diagram (diagram 2.1.).
System of verbal and visual teaching methods
This division into blocks is determined by the content of the chemistry course. A demonstration experiment and natural objects help to study the properties of substances and the external manifestations of a chemical reaction. Models, drawings, graphs (this also includes the compilation of formulas and chemical equations as symbolic models of substances and processes) help explain the essence of processes, the composition and structure of substances, and provide a theoretical justification for observed phenomena. This division of visualization functions indicates the need to use the content of both blocks in didactic unity.
Didactic unity is reflected in the so-called equipment complexes on the topic. The chemical process in the device occurs under certain conditions. To justify them, you can provide reference data on substances in the form of graphs or digital data, explain the process using ball-and-stick models, etc. It is important not to get carried away with too much visualization, as this tires students.
Particular attention should be paid to the combination of visualization with the teacher’s word. Experience shown without a teacher’s commentary is not only not beneficial, but sometimes can even be harmful. For example, when demonstrating the interaction of zinc with hydrochloric acid, students may get the impression that hydrogen is released not from the acid, but from the zinc. A very common mistake is the opinion that it is not the indicator that changes color, but the environment into which it enters. And most other experiments without explanation will not perform the necessary educational, nurturing and developmental functions. Therefore, the teacher’s word plays an important guiding and guiding role. But the word is also in a certain dependence on the means of visualization, since the teacher builds his explanation, focusing on the means of teaching that are at his disposal.
Using a demonstration experiment in teaching chemistry
The most important of the verbal and visual teaching methods is the use of a demonstration chemical experiment. The specificity of chemistry as an experimental-theoretical science has placed the educational experiment in one of the leading places. A chemical experiment in teaching allows students to become more familiar not only with the phenomena themselves, but also with the methods of chemical science.
A demonstration is an experiment conducted in a classroom by a teacher, a laboratory assistant, or sometimes one of the students. Demonstration experiments in chemistry are indicated in the program, but the teacher can replace them with others that are methodologically equivalent if he does not have the required reagents.
The teacher uses a demonstration experiment at the beginning of the course, when students do not yet have skills in chemistry, in order to teach them to observe processes, work methods, and manipulations.
This is done to arouse interest in the subject, begin the formation of practical skills, familiarize them with the appearance of chemical glassware, instruments, substances, etc. A demonstration experiment is used when it is too complex for students to perform independently (for example, the synthesis of sulfur oxide (VI) from oxide (IV) and oxygen) if it is dangerous when performed by students (for example, an explosion of detonating gas). A demonstration experiment is necessary if it has methodological value when working with a large number of substances, since with small quantities it is not convincing enough (for example, extinguishing burning gasoline or alcohol with carbon dioxide).
The problem of using a school chemical experiment is one of the most developed in the methodology, since it is this that more than others reflects the specifics of the educational subject. Widely known in the research methodology are V.N. Verkhovsky, K.Ya. Parmenov, V.S. Polosin, JI. A. Tsvetkova, I. N. Chertkova, A. D. Smirnova, I. JI. Drizhuna et al. Materials about chemical experiments are regularly published on the pages of the journal “Chemistry at School”. The requirements for a demonstration experiment are well known.
Visibility. Visualization is the most important principle of learning, proclaimed by J. A. Komensky. It is no coincidence that popular wisdom says: “It is better to see once than to hear a hundred times”; It is generally accepted that the visual channel of information is the most effective. So the demonstration of experiments is intended to provide visibility of the processes.
Reagents should be used in such quantities and in containers of such volume that all parts are clearly visible to all students. Test tube experiments are clearly visible no further than the third row of tables, so cylinders, glasses or demonstration tubes of sufficiently large volume are used for demonstration. Anything that might distract attention is removed from the table. The teacher's gesture should be carefully thought out; the teacher's hands should not obscure what is happening.
The clarity of the experiment can be enhanced by demonstrating it through a graphic projector in a cuvette or Petri dish. For example, the interaction of sodium with water cannot be shown with a large amount of metal, and with a small amount it is poorly visible, and it cannot be given to students for laboratory work - the experiment is dangerous. An experiment illustrating the properties of sodium is very clearly visible when projected through a graphic projector. For greater clarity, stage tables are widely used.
Simplicity. There should be no clutter of unnecessary parts in the devices. It should be remembered that, as a rule, in chemistry the object of study is not the device itself, but the process occurring in it. Therefore, the simpler the device itself, the better it meets the purpose of learning, the easier it is to explain the experience. However, simplicity should not be confused with oversimplification. Do not use household utensils in experiments - this reduces the culture of the experiment. Students watch spectacular experiments with flashes, explosions, etc. with great pleasure, but they should not get carried away with them, especially at the beginning of their studies, since less effective experiments will receive less attention.
Experimental safety. The teacher bears full responsibility for the safety of students during class and in extracurricular activities. Therefore, he must know the safety rules when working in a chemical laboratory. In addition to providing classes with fire safety equipment, exhaust means, and means for providing first aid to victims, the teacher needs to remember the techniques that promote safety in the lesson. The containers in which the experiment is carried out must always be clean, the reagents are checked in advance, and for experiments with explosions a protective transparent screen is used. Gases are checked for purity in advance and before the experiment itself. If the experiment is carried out with an explosion, students are warned about this in advance so that the explosion does not come as a surprise to them. Work with toxic gases is carried out in a fume hood. All this is important for students’ environmental education.27
In recent years, special equipment has been developed for conducting experiments in closed systems. This allows you to work with poisonous gases without draft.
It is necessary to provide personal safety equipment (safety glasses, a cotton robe, rubber gloves, a gas mask, etc.), and ensure that the hair is tied up.
Reliability. The experience should always be successful, since a failed experience causes disappointment in students and undermines the authority of the teacher. The experiment is checked before the lesson in order to work out the technique of conducting it, determine the time it will take, find out the optimal conditions (sequence and quantity of added reagents, the concentration of their solutions), think over the place of the experiment in the lesson and the explanation plan. If the experiment still fails, it is better to immediately show it again. The reason for failure should be explained to students. If the experiment cannot be carried out again, then it must be shown in the next lesson.
The need to explain the experiment. Each experiment only has educational value when it is explained. Fewer experiments in a lesson are better, but all of them should be understandable to students. According to I. A. Kablukov, students should look at experience as a method of studying nature, as a question asked of nature, and not as “hocus pocus.”
Execution technique. The most important requirement for a demonstration experiment is the filigree technique of its implementation. The slightest mistake made by the teacher will be repeated many times by his students.
In accordance with the listed requirements, the following methodology for demonstrating experiments is recommended.
Setting a goal for the experiment (or a problem to be solved). Students must understand why the experiment is being carried out, what they must be convinced of, and what they must understand as a result of the experiment.
2.
Description of the device in which the experiment is carried out, the conditions under which it is carried out, the reagents, indicating their required properties.
3.
Organization of student observation. The teacher should orient students which part of the device to observe, what to expect (a sign of a reaction), etc.
It is very important to avoid a number of mistakes common to beginning teachers. You cannot tell students what they should see. For example, if during the experiment the color of the solution turns crimson, the teacher should not say this in advance. But you need to tell students where to focus their attention by saying, “Watch to see if the color of the solution changes.” If the color should change, but does not change, you should not convince children that “the change, at least a little, happened.” It is necessary to indicate where to look, in which part of the device the main process that needs to be observed should take place. For example, when oxidizing S02 to S03 on the catalyst Cr2O3, it is necessary to prove that S03 was actually produced. The experiment is carried out in the device, the resulting S03 is taken into the receiving flask, where the BaCl2 solution is located. During the reaction of BaCl2 with S03, a white precipitate gradually forms. When observing, this is exactly what you need to catch, but the attention of students is much more attracted to the calcium chloride tube with a green catalyst, where no external changes occur. 4.
Conclusion and theoretical justification.
To master a chemical experiment well, you need repeated and lengthy practice in conducting it.
In the process of demonstration, three functions of the educational process are carried out: educational, nurturing and developing: -
the educational function is expressed in the fact that students receive information about the course of chemical processes, the properties of substances, and the methods of chemical science;
-
D. M. Kiryushkin and V. S. Polosin discovered the following pattern. If the teacher's word precedes the experience, then the demonstration is illustrative. If the word follows the showing of experience, then problematic.
For example, showing a “fountain” when hydrogen chloride is dissolved in water, you can first talk about its high solubility in water, and then show experience as confirmation of your words. Or you can first show the experience, and then require students to explain themselves, stimulating their search activity. However, conducting problem experiments is not at all limited to observing the sequence of words and experiments. Everything is much more complicated. For a detailed study of this important problem in the methodology, it is useful to read the book by Yu. V. Surin.28
Four ways of combining the teacher’s word with the experiment have been identified.29 1)
knowledge is derived from experience itself. The teacher’s explanation accompanies the experience and goes, as it were, parallel to the process that the students observe. This combination is unacceptable for spectacular experiments that attract the attention of students with a bright spectacle and create a strong dominant focus of excitation in the cerebral cortex;
2)
the teacher’s word complements the observations made in the experiment, explains what the students see (for example, an experiment with the reduction of copper from oxide with hydrogen);
3)
the teacher's word precedes the experiment, which serves an illustrative function;
4) first a verbal explanation is given, a decoding of the phenomenon, and then a demonstration experiment. However, it does not follow from this that when demonstrating, the teacher predicts the course of the experiment and tells what should happen.
The first and second approaches are used for problem-based learning; they are more conducive to the development of mental activity.
Recently, on-screen aids, which are important visual aids, have been actively used. To demonstrate them, technical means are needed: a film camera, overhead projector, epiprojector, graphic projector, video recorder, television, etc. These technical means themselves do not have educational properties and are not objects of study in chemistry lessons, but without them the use of on-screen aids is impossible. When working with the screen aid, students receive many figurative ideas.
For on-screen aids, it is necessary to determine a place in the complex of visual aids, if possible, organize a discussion as they are demonstrated, combining the aid with the teacher’s word and trying to provide feedback, to use the capabilities of on-screen aids in the education and broadening of horizons, and the development of students. Methods for using on-screen aids, as well as other visual aids, depend on the didactic purpose and content of the educational material.
The methodology for using on-screen aids is described in particular detail in the JI book. S. Zaznobina.30
Writing on the board needs to be planned in advance. It must be performed clearly and consistently, so that the entire course of the lesson is reflected on the board. In this case, the teacher can return to what has already been explained and discuss with the students questions that are not well understood. Drawings on the board are made using stencils.
The teacher also supervises the students’ work at the blackboard so that their writing is clear and accurate.
Writing on the board is more appropriate than other types of visualization in cases where you need to reflect the sequence of derivation of a formula or other algorithmic prescription. You should only use a clean board that has no extraneous notes on it. The teacher should stand at the board so as not to block the note he is making.
In some cases, notes on the board can be replaced by magnetic applications, applications on flannelograph, etc. Tables, diagrams, graphs, etc. are widely used for various didactic purposes. The table can depict a production installation, show a laboratory technique, or a graphic model molecules or crystal lattice, etc. The value of the tables is that they can be presented to students at any time. They are used at any didactic stage of the lesson - to study new material, when consolidating and improving knowledge, when testing knowledge.
Literature on the topic 1.
Verkhovsky V.N., Smirnov A.D. Technique and methodology of chemical experiment at school. In 2 volumes - M.: Education, 1979. 2.
Zaznobila L. S. Screen aids in chemistry lessons. - M.: Education, 1990. 3.
Kiryushkin D. M., Polosin V. S. Methods of teaching chemistry. - M.: Education, 1970. 4.
Konovalov V.N. Safety precautions when working in chemistry. - M.: Education, 1973. 5.
Mauriva I. Ya. Systematic approach to creating educational tables in chemistry. - M.: 1974. 6.
Nazarova T. S., Grabetsky A. A., Lavrova V. N. Chemical experiment at school. - M.: Education, 1987. 7.
Parmenov K. Ya. Chemical experiment in high school. - M.: APN RSFSR, 1959. 8.
Parmenov K. Ya. Demonstration chemical experiment. - M.: APN RSFSR, 1954. 9.
Parmenov K. Ya., Safonova I. N., Teterin M. L. Experimental work of students in chemistry. - M.: APN RSFSR, 1952. 10.
Pletner Yu. V., Polosin V. S. Workshop on methods of teaching chemistry. - M.: Education, 1981. 11.
Polosin V.S. School experiment in inorganic chemistry. - M.: Education, 1970. 12.
Polosin V. S., Prokopenko V. G. Workshop on methods of teaching chemistry. - M.: Education, 1989. 13.
Semenov A. S. Occupational health and safety. - M.: Education, 1986. 14.
Tsvetkov L. A. Experiment in organic chemistry. - M.: Education, 1986. 15.
Chertkov I. N. Experiment on polymers in high school. - M.: Education, 1980. 16.
Chertkov I. N., Zhukov P. N. Chemical experiment with small quantities of reagents. - M.: Education, 1989.

LET'S LOOK INTO THE HISTORY OF THE QUESTION Y.A. Kamensky “transformed” the observation method into a teaching method. Comenius considers perception (observation) as the source of all knowledge, since he assumes that things are directly imprinted in the mind and only after becoming familiar with the thing itself should explanations be given.

The problem of visibility was presented even more broadly and more justifiably in the works of I.G. Pestalozzi. If for Comenius observation (visibility) serves as a child’s way of accumulating knowledge about the world around him, then for Pestalozzi visibility acts as a means of developing the child’s abilities and spiritual powers.

The problem of visibility in pedagogy was analyzed comprehensively and deeply by K.D. Ushinsky. When asked what visual teaching is, Ushinsky answers this way: “This is a teaching that is built not on abstract ideas and words, but on specific images directly perceived by the child.”

The process of cognition according to Ushinsky consists of two main stages: 1) sensory perception of objects and phenomena of the external world; 2) abstract thinking. He sees the essence of visual learning in helping, with the help of visual aids or real objects themselves: – the formation in children of a clear and clear idea of objects and phenomena; – identifying connections between objects and phenomena; – formation of a certain generalization.



Currently, pedagogy connects visual learning with the following features: – the correct use of visualization depends on its accompaniment by the teacher’s word; – visual aids can be effective if the student has some experience working with the object being studied; – for effective assimilation of knowledge, visualization alone is not enough – it must be supplemented by the active activity of the student himself.




1.Use of electronic multimedia textbooks (CDs and DVDs) in the classroom One of the areas of modernization of the education system at school is the introduction of computer and multimedia technologies. Multimedia discs can significantly save time, both in class and during preparation of material. The computer becomes a faithful assistant for students and teachers. It allows you to accumulate and preserve a didactic base and solve the problem of visibility.

“Types of activities in chemistry lessons” - Teaching in small groups. Didactic games. Problem-developing experiment. Using IT in chemistry lessons. Integral cognitive tasks. Group technology. Active forms of classes. Information Technology. Activation of cognitive activity in chemistry lessons. Types of didactic games.
"Chemical equipment" - Scales. Test tubes. Wurtz flasks. Evaporation cups. Graduated cylinders. Chemical beakers. Books. Tripods with a set of holders. Mortars. Buchner funnels. Heating the test tubes. Conical flasks. Burettes. Dephlegmators. Metal equipment. General purpose utensils. China. Special purpose utensils.
“School chemistry classroom” - Chemistry lessons are held in the classroom from grades 8 to 11. Chemistry room. Priority of independent activity. Finding ways to improve the lesson. Storage of reagents and equipment. Use of information technology. The main goals of the office. Selection of educational, reference and popular science literature.
“Chemistry knowledge test” - Type of chemical bond in a hydrogen molecule. Divide the mixture. What does chemistry study? Theoretical workshop with demonstration of experiments. Which device is called the Kipp apparatus? Non-metals. Which device is prepared for producing hydrogen? Giant enterprises. Unscramble the abbreviation. Determine where the potassium carbonate was located.
“Chemical and biological class” - Determination of qualitative and quantitative composition. Methods and forms of implementation of the variable component of the school course. Pre-professional elective courses. Contents of a specialized chemistry course. Examples of profile-oriented components of a chemistry course. Project and research work of students. Elective courses for chemical and biological classes.
“Safety in chemistry” - General safety requirements. Student Instruction Journal. Safety precautions in the chemistry classroom. Types of instruction. List of instructions. Healthy learning environment. Transaction log. List of instructions on safety rules. List of documents. Drug precursor. List of chemicals.
There are 19 presentations in total
Chemistry is a very exciting discipline, but, unfortunately, not every educational institution can boast of a well-equipped chemistry classroom, so, unfortunately, all experiments are often carried out in words. Chemistry presentations will help the teacher show students various interesting chemical experiments, which will help the knowledge to be absorbed much better. With a large number of slides and videos, the presentations will become an indispensable tool in many chemistry classes.
Chemistry presentations are made in PowerPoint; here you will find a large assortment of chemistry presentations that can be downloaded absolutely free. To do this, you need to go to the selected presentation and click on the “download” button. Before this, you can see each slide and view their description. You don’t have to download the files first and only then realize that this is not exactly what you need. If you have difficulty finding the topic you need, you can use the search for all presentations, enter a keyword and we will select the most suitable work for you.
Here you will find presentations on chemistry for both elementary and high school students. Thanks to the clarity, colorfulness of the slides, correctly structured and divided into blocks of information, the audience will be able to more easily perceive the subject and better concentrate on the topic.