Oxidation of terminals - a similar problem occurred in almost every experienced motorist and not only. In fact, such a terminal is covered with a coating that prevents the battery from working correctly. As it accumulates, plaque can lead to the fact that the car does. And this is especially true in winter - in cold weather. Sometimes oxidation is so intense that plaque completely covers the terminal.
White bloom on the terminals is a sure sign of oxidation.
That is why contacts need to be cleaned periodically. But eliminating only the consequences of the problem will not solve. It is also necessary to look for the cause of this phenomenon.
Causes of terminal oxidation
1. Leakage of filled electrolyte is by far the most common problem. The electrolyte is an acid that, when it hits the surface of the terminal, enters into a chemical reaction with it, as a result of which oxidation begins.
As a rule, such problems are uncommon. After all, these are closed-type batteries, in which the electrolyte is in a sealed container, where it evaporates and precipitates. However, the battery is designed to prevent it from being released into the environment.
However, as the battery wears out, its body cracks and electrolyte begins to escape into these microcracks in the form of steam. So it settles directly on the terminal, as a result of which a reaction begins. A similar phenomenon should be considered by the car owner as a sign of malfunction and deterioration of the battery.

If the battery terminal is not fully tightened, this can cause its oxidation.
2. Tightening the terminal - such situations periodically arise. This means that the owners of the car simply put a terminal on the electrode, after which they immediately sit behind the wheel without tightening it with bolts. Insufficient quality fixation leads to poor contact and oxidation at the electrode and terminal. In this case, it is enough to remove the terminal, clean both it and the electrode with high quality, then put it on and tighten it well. Only not with all your strength, otherwise you can rip off the fasteners.
In this case, it is necessary to wield 2 keys at once, one of them, and the other to hold the bolt. But it is not recommended to pull with all force on one side, because this is fraught with deformation of the plastic. You should also be wary of another common problem - the gradual loosening of the pin termination. This occurs as a result of poor-quality dismantling of the clamps from the wires. Therefore, you need to accurately dose the effort.
Oxidation effects
They manifest themselves in a noticeable deterioration of the spot and the quality of the contact. As a result, the battery is unable to charge and operate properly. Subsequently, this will lead to serious discharges of the battery, which affects it extremely negatively. In addition, this is reflected in a complicated, if not completely lack of response to the turn of the key.
Ways to combat oxidation of battery terminals
Emery cleaning
In general, the method is simple and straightforward. It can be done with a wire brush or sandpaper. First you need to remove the terminal from the electrode. Complications may arise with this, because the formed plaque interferes with work. Further, armed with sandpaper (but only fine) or a metal brush, it is necessary to thoroughly clean the place of contact between the electrode and the terminal. Particular attention should be paid to the inner surface of the terminal. It is required to clean to a shine, but also not to overdo it.
Video: How to lubricate the battery terminals?
Petrol
Part of motorists to remove plaque. The cloth is moistened with it, after which the terminals and electrodes are wiped until the plaque is removed. However, using this method requires caution. After all, gasoline belongs to the category of flammable liquids. And it is also necessary not to allow it to come into contact with plastic or rubber components, because gasoline is a solvent. Consequently, it can damage plastic and rubber.
Methods to combat oxidation
First, it is required to clearly determine whether electrolyte passes through the electrodes. If the answer is yes, it is necessary to exclude this factor.
Replacement
This is the most radical method of solving the problem. However, not everyone is ready to go straight for a new battery pack. However, this method is the only one if there is a break in the electrode attachment. In this case, the passage of electrolyte is guaranteed.
Something else useful for you:
Insulation
To do this, you can use both the proven "old-fashioned" method and modern solutions.
Video: Oxidizing Battery Terminals
Many car owners know that if you arm yourself with a felt ring and soak it with engine oil, you can successfully solve the oxidation problem. As a result, the formation of an oiled layer occurs, due to which evaporation of the electrolyte is excluded, and no plaque appears on the contacts. The implementation of this method is extremely simple - you need to put one ring of felt soaked in oil on the battery electrode, then fix the terminal, and put another ring on top. But engine oil isn't your only option. Instead, it is allowed to use technical petroleum jelly, solid oil or other insulating substance.
You can go in a more modern way. It involves the use of not a simple motor oil, but a special impregnation - "electric fat". A similar tool was originally developed to protect the battery terminals.
Outcome
In order to prolong the successful and uninterrupted functioning of the battery, it is necessary not only to combat oxidation and plaque formation on the terminals, but also to eliminate the cause of this phenomenon in time. All this will greatly facilitate starting the engine in cold weather.
Almost every car enthusiast has heard or personally encountered such a concept as oxidation of the battery terminals. The essence of this phenomenon lies in a chemical process that causes a characteristic plaque and makes it difficult for the battery to function normally. In this article, we will talk about the causes and consequences of this unpleasant process, as well as give some valuable advice. Almost every car enthusiast has heard or personally encountered such a concept as oxidation of the battery terminals. The essence of this phenomenon lies in a chemical process that causes a characteristic plaque and makes it difficult for the battery to function normally. In this article we will talk about the causes and consequences of this unpleasant process, as well as give some valuable advice.
So, battery oxidation and its terminals is a process that sooner or later occurs in any car battery. This occurs most often in cold and humid weather, which promotes the spread and increase in the intensity of the chemical reaction on the contact surface. You ask, what does this state of the battery promise? The result of oxidation is complete or partial vehicle failure. It will simply stop starting, and you will not be able to continue operating the car.
In order to get rid of plaque on the battery terminals, it is enough to clean with a special brush, and also lubricate the contacts with antioxidant grease. However, sometimes this will not be enough, because the root of the problem may lie in a completely different place.
Reasons for oxidation of battery contacts:
1. A common and easily corrected cause is poor contact between the terminal and the battery electrode. Car enthusiasts very often simply put the terminal ring on the battery and start operating the car, without even thinking that it is necessary to carry out the appropriate tightening with bolts. In this case, you just need to remove the terminal and clean it with a brush, then put it on and tighten it properly. Try not to use excessive force to avoid ripping off the threads.

2.
A common cause of battery oxidation is leakage or leakage of electrolyte, the acid inside the battery. Possessing certain properties, it gets on the metal of the terminal and initiates a chemical reaction, which results in a characteristic white coating. If this happens, then you need to think about replacing the battery, because its tightness is broken. New battery negates various electrolyte leaks. 
IMPORTANT: the consequences of oxidative processes in the battery is the difficult start of the engine, due to the weak contact between the terminal and the electrode. The contact area is significantly reduced, and the resistance also increases, which does not allow the current to flow normally in the car's electrical system.
Conclusion
If you are faced with the fact that the car does not start and the indicators on the devices are poorly lit, first of all check the quality of the contacts on the battery, and also carry out the maintenance of your iron horse in a timely manner.
To avoid such a problem, you should periodically review the contacts and, if necessary, clean them. However, simply cleaning the terminals does not always solve the problem, so it is best to look for the root cause of oxidation.
Reasons for oxidation of battery terminals
If you have such a problem, the first step is to find the reason why the terminals on the car battery are oxidizing. There may be several reasons:
- The development of the battery life when the seal rods are already dry.
- Electrolyte leakage. The most common reason. Since the electrolyte is an acid and its contact with the contacts is associated with a reaction, as a result of which the oxidation process occurs.
- An electrolyte that has an invalid density reading. To avoid such situations, when replacing it, you need to use only the ready-made composition. It is not recommended to independently measure the components to the established proportions.
- Tightening the terminal. Loose attachment of the electrode to the terminal gives poor contact, causing a reaction. This situation can be corrected by cleaning the electrode and terminal. Put everything in place and tighten it well, but do not overdo it, as there is a chance of ripping off the fasteners.
Experienced drivers have encountered this problem more than once, so they can easily explain why the positive or negative terminal of the battery is oxidized.
Most often, the negative terminal of the battery is oxidized. The main cause of oxides is battery wear. Microcracks begin to appear in its body, from which electrolyte is actively oozing, the vapors of which turn into oxides.
In this case, the negative terminal must be periodically cleaned to a shine, although most drivers remove only oxides without touching the lead. This is wrong, because if the contacts are unscrewed, then they must be processed in the most careful way.
Why is the positive terminal on the battery oxidized? There are just two answers:
- Systematic recharging of the battery, as a result of which the electrolyte overheats and begins to evaporate.
- Somewhere there was a violation of the tightness of the battery case, which requires prompt intervention in the situation, since the acid can even eat through the case over time.
Oxidation of battery terminals - symptoms and signs
One of the most obvious and common signs of terminal oxidation is the dim, not bright light of headlights, side lights, brake lights, turn signals when the battery is fully charged. It is also possible to oxidize the terminal if, if necessary, start the car and the starter does not "grab" the first time or turns the crankshaft very hard, as if the battery is discharged, although in reality it is not.
How and how to clean the battery terminals
The white coating on the battery terminals is oxidized lead, which must be wiped off for the electrode and contact to interact. Since these chain elements are metal surfaces, they are intensively cleaned without fear of damage and without following special rules.
There are many ways to clean the battery terminals. It is possible to quickly and effectively remove white plaque both with chemical reagents available in the retail network and with improvised means. The most effective and affordable are the following methods:
- Sandpaper. Turn off the engine and remove the key from the ignition before stripping the terminals and electrode. So that this process does not take much time, it is better to use sandpaper with the largest grain size for these purposes. This method is quite simple, and sandpaper perfectly erases traces of oxidation and almost everyone has it. It is necessary to clean the electrode and terminal to a shiny state.
- You can use a soda solution to remove the remaining acid from the terminals. If the acid is in contact with metal, small bubbles will appear during the processing of the terminals. The ratio of soda and water: 1 tbsp. spoon to glass. After rinsing with such a solution, the remains of soda must be removed from the terminals; for this, it is enough to wipe them with a damp cloth.
- Petrol. Less convenient method of cleaning the electrode and terminals from oxidation. Gasoline corrodes oxides well and quickly, but there is a possibility of it getting on rubber or plastic components, which can negatively affect their strength. If you decide to clean the terminals in this way, you need to moisten a rag with gasoline and gently rub the terminals until all traces of oxidation are removed.
Important! It is highly undesirable to use WD cleaners to clean terminals and electrical contacts. In addition to oil, they contain aggressive conductive cleaning agents of unknown composition to the purchaser. Also, acetone should not be used, as it even corrodes metals.
Effective methods for protecting battery terminals
1) Litol, solid oil. To protect the terminals from aggressive effects, solid lubricants have long been used as the main materials - solid oil or lithol, which are non-conductive. As a result of the processes of expansion of contacts, under the influence of temperature, these lubricants penetrate into the intercontact zone, and with time practically into all cavities of the contact zone.
Moreover, in the process of natural wear and tear, as well as with significant temperature changes, the technical characteristics of solid oil and lithol change - they harden. If you decide to use lithol or solid oil, it is recommended: when cleaning the contact twice a year, a complete replacement of the grease is necessary (removing the old one, applying a new one).
2) Silicone grease. Ideal for handling terminals and electrical connections. However, when buying it, you need to pay attention that there are no conductive additives in the composition (as a rule, manufacturers warn about this).
Such a lubricant not only delays the impact of an aggressive environment, but also repels it. The battery terminals are processed exclusively in complex application with other preventive materials. It is applied to a clean treated surface, after which they begin to connect the parts.
This grease has one drawback that is significant for the processing of battery terminals - fluidity. Silicone grease leaves contact over time and must be injected regularly during use.
3) Special means. Now on sale there are many tools designed specifically for processing battery terminals. Their tubes have detailed instructions for use. Undoubtedly, such funds are better than using solidol or other questionable substances. They are based on a protective oil environment and, as a rule, it is liquid paraffin. How to lubricate the battery terminals so that they do not oxidize is the choice for the car owner. All means and the above methods are equally good, it remains only to choose the most acceptable one.
Battery maintenance

To prevent oxidation of the battery terminals from causing a sudden stop of the vehicle, the following work should be carried out regularly:
- external inspection of the surface and contacts of the battery once a month;
- if traces of oxidation are found on the terminals, immediately check the tightness of the battery;
- in the warm season, check the electrolyte level in serviced batteries once a month;
- once every 3-4 months (more often in summer and winter) wipe the battery surface with a dry cloth;
- before the start of the winter season, it is imperative to check the condition of the terminals (how hot they heat up during the start of the motor "to cold"), even if there is slight heating, the contacts should be checked and further processed.
It should be remembered that battery problems are a common cause of engine start failure during cold seasons. Often this reason lies precisely in the oxidation of the battery terminals. Protecting the battery terminals from oxidation is an important process, so regular monitoring and preventive measures will help avoid this situation.
The habit of lubricating battery terminals from oxidation was inherited from our grandfathers.
Let's try to figure out how effective this protection measure is, whether it is worth using it on modern car batteries in general.
Why do the battery terminals oxidize?
It is not entirely correct to call the process of the formation of foreign plaque and other manifestations of destruction of the surface and contacts of the battery terminals purely oxidation. In general, it is a collection of several chemical processes. Let's define the main ones.
Natural oxidation
All substances in the air, where there is a lot of oxygen, enter into the oxidation process, or oxidation. Lead, namely, the battery terminals are made of it, from this point of view, is not very susceptible to oxidation, there are "weaker" metals, take the same copper.
However, we can only observe unoxidized lead (shiny) when stripping the battery contacts. Within a few minutes, it begins to darken, that is, to oxidize.
There is nothing wrong with the natural oxidation of lead, the lead oxide film is very thin, it is easy to destroy it by slightly moving the terminal. In addition, it is almost impossible to allow 100% protection from oxygen, except by placing the battery in a pressure chamber. Even such thick lubricants as antifreeze and grease allow oxygen particles to pass through.
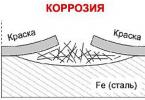
Acid destruction
This type of chemical reaction leads to the formation of a "snowy" white coating on the battery terminals. In addition to a not quite aesthetic appearance, the plaque introduces additional transition resistance at the point of contact, leads to the destruction of the terminals, and can cause leaks.
Where does acid come from? In most cases from the battery. Serviced batteries have technological holes for filling electrolyte and monitoring its level.
Video - what to do if the battery terminals are oxidized:
Almost all car batteries are equipped with a gas evacuation system. If these zones are leaking (the gas outlet is always leaking if the gas outlet pipe is not connected to it), electrolyte vapors are released through them during operation.
This is especially evident in the warm season, although under the hood of a car it occurs even in winter after twenty minutes of driving.
The process of overheating the battery, especially boiling, is even more terrible from the point of view of acid emission. This is possible in the following cases:
- destruction of the plates of the battery cells;
- short circuit of battery cans;
- wrong (overcharge);
- lack of natural cooling of the battery during the warm season.
The white deposits that appear on the battery terminals represent lead chloride. This is a rather corrosive environment, especially for electrical connections. If it is not removed in time, it will "eat" the contact in a couple of months, and in such a way that it may not be subject to recovery.
Salt destruction
Russian highways are still sprinkled with sodium and potassium salts in winter. The composition of modern anti-icing equipment is a trade secret, at least for motorists.
But all mixtures with water vapor and impurities of other substances on the road, ultimately, envelop all parts of the car, including the battery contacts.
Salt plus water is an excellent electrolyte, and where there is electrolyte, metals and electrical voltage, there is an electrolysis process. It slowly destroys metal parts.
Electrolysis is not capable of destroying the massive battery terminal, but it is possible to make a change in the contact zone.
Cleaning as a method of struggle
The simplest and most reliable method is the timely removal of plaque, cleaning the battery terminals.
Technical advice: no matter what means you protect the battery terminals, at least every six months (preferably at the borders of the winter and summer seasons), clean the terminals mechanically.
This will save you from problems with starting the engine, leakage processes and heating of the contacts. In order to clean them, it is necessary to unscrew the crimp bolts, preferably completely, disconnect the terminals. Then moisten them with an alcohol-based liquid and wipe dry.
If this is not available, a solvent diluted with water can be used. So it is more fireproof, in addition, the aggressiveness of the solvent decreases.
To remove possible residues of ingrained acid from the terminals, it is necessary to use a soda solution (a tablespoon of soda in a glass of water). If the acid did come into contact with the metal, small bubbles will appear during the treatment with a sponge or rag soaked in the solution. After rinsing with soda solution, wipe the terminals with a damp cloth.
Under no circumstances should WD cleaners be used for cleaning. In addition to oil, they contain conductive aggressive cleaning agents of unknown composition to the consumer. WD tools are highly undesirable for handling electrical contacts.
Also, acetone cannot be used to process electrical contacts, it corrodes even metals.
Further cleaning is carried out using fine sandpaper, it is possible on a paper basis. Pretreating heavily soiled terminals can be done with a wire brush. There are special brushes on sale for such work.
Video - how you can clean the battery terminals:
The ideal work result is a shiny surface, not necessarily perfectly flat. Lead is a soft material, so at the moment the terminals are crimped, the contacts will get maximum contact.
After carrying out mechanical cleaning work, a natural question arises: how to maintain the ideal state of contacts in the period between maintenance work? The answer is simple: to protect the terminals from the ingress of corrosive substances using various materials.
For this, various lubricants and other materials are used.
How to lubricate the battery terminals from oxidation
Litol, solid oil
For a long time, solid lubricants have been used as the main materials for protection against aggressive chemical effects: lithol, grease, grease.
To protect the battery terminals, mainly solid oil or lithol was used. These are non-conductive lubricants.
As a result of the processes of thermal expansion of contacts and mechanical joints, these lubricants gradually penetrate into the intercontact zone. Over time, they penetrate into almost all internal cavities of the contact zone.
In addition, at large temperature differences, as well as in the process of natural wear and tear, the technical characteristics of lithol and solid oil change: they harden. As a result of this, electrical contact may be broken at once.
Recommendations for those who decide to use lithol or solid oil: when carrying out routine maintenance to clean the contact (at least twice a year), a complete lubricant change is required, i.e. removing old and applying new grease.
Silicone Grease
Excellent lubricant in all respects for carrying out the processing of electrical connections. But only when buying it should you pay attention that it is free of conductive additives (manufacturers usually warn about this on the bottle).
Lubrication differs in that it not only traps aggressive media, it repels them. It's like a fur coat that repels cold.
For the processing of battery terminals, the grease is only suitable for complex application with other materials. It is applied to the treated clean surfaces, then proceeds to the mechanical connection of the parts.
The main disadvantage of grease in terms of handling battery terminals is fluidity. Over time, the silicone grease leaves the contact.
If you use silicone grease, it is necessary to inject it regularly, then remove the grease that has not got into place from the contact area with a rag. A bit of a hassle.
Graphite grease
Some motorists used graphite grease to process the battery contacts, justifying its use with conductive properties. However, this grease has a high resistivity and, in the absence of metallic electrical contact of the terminals, passing a high current through itself, can lead to heating and even ignition.
Apply graphite grease to the battery terminals it is forbidden!!!
Special means
Many products are now produced specifically for the treatment of car battery terminals. We will not describe the benefits of using specific brands, manufacturers do a good job with this themselves.
Video - how can you lubricate the battery terminals so that they do not oxidize (MS-1710 tool):
The tubes of these treatments have instructions for their use. Of course, such funds are better than grandfather's grease, although they are also based on an oily medium, as a rule, vaseline oil.
Spray lubricants are very convenient.
The process of applying protective equipment to the battery contacts
Having decided on the material for the protective coating, they begin to apply it. To do this, first perform mechanical cleaning (see above).
After the terminals are cleaned, it is best to pre-treat the contact areas with silicone grease.
Which is better? This is where the opinions of auto electricians differ. The first is supported by the fact that under the terminal, in any case, there are air cavities, if grease gets into these places, it will only be better.
However, if you make frequent disconnection of the terminals, for example, to recharge the battery, it is better not to apply grease under the terminals.
When using branded lubricants, the instructions will indicate how to apply them.
Car batteries are operated in rather harsh conditions associated with large temperature differences, vibration, exposure to corrosive environments and other adverse factors. In this regard, they often have various problems and problems. One of the most common problems with car batteries is the oxidation of their contact terminals.
Curbing
Reasons for oxidation of battery terminals
Problems associated with poor contact are the most common cause of damage to any electrical equipment. The appearance of oxides on the contacts of the battery is harmful to the electrical equipment of the car and may indicate a problem in the battery.
A small oxide on metal surfaces inevitably appears as a result of the interaction of their surface with atmospheric oxygen and other reagents. Therefore, even a very high-quality electrical contact deteriorates over time due to natural oxidation processes. In addition, if the contacts are made of different metals, a galvanic potential difference arises between them, also leading to premature oxidation of the surface.
With the appearance of additional aggressive factors or improper fixation of the terminals, conduction disturbances and oxides of the contact pads can very quickly occur.
Excessive oxidation of car battery terminals typically occurs for four reasons:
- corrosive effect when electrolyte vapors leak from the damaged battery case (electrochemical corrosion) - such an oxide is white;
- poor contact at the junction of the battery terminals with the car's conductors due to weak tightening, high humidity in the engine compartment and dirt entering the unsecured gap, which leads to arcing and burning of the contact point, leading to blackening;
- moisture in the engine compartment oxidizes the copper contained in the terminal - because of this, a greenish coating and rust appears;
- the battery contact is made of lead, and the car wiring conductor is made of another metal (copper or brass), which leads to a chemical reaction between them - the color of the oxide may be greenish.
Oxides that appear for these reasons can appear on both battery electrodes. Due to the small design differences of these contacts, the frequency of their appearance on each electrode is slightly different.
Why is the positive terminal on the battery oxidized?
The positive terminal on most cars is covered with a plastic cover, which contributes to the accumulation of condensation under it. In case of poor tightness of the battery from exposure to high temperatures during engine operation, as well as when it is severely overcharged, electrolyte vapors leak. Acid particles from these vapors concentrate over time under the insulating cover in the area of the positive terminal and lead to its oxidation.
At the same time, due to the fact that the positive electrode is covered with a lid, less external contamination gets into the junction, which reduces the likelihood of poor contact, arcing and associated blackening.
Thus, on the plus side, the appearance of a white oxide associated with the leakage of electrolyte vapors is likely.
Why is the negative terminal of the battery oxidized?
The negative terminal of the battery is more susceptible to external influences because it does not have a protective cover. Because of this, acid vapors of the electrolyte condense less on it, but more dust and dirt gets into it. All this leads to a more rapid deterioration of the electrical contact of the battery cathode with the wiring, the appearance of small spark discharges during engine starts, which ultimately lead to burnout and blackening of the contact surface.
These problems most often appear when connecting a battery with initially dirty contacts and when tightening them loosely.
What can lead to oxidation of the terminals
Over time, the resistance at the site of the oxidized contacts becomes so great that most of the battery current is lost on it, and the starter cannot rotate. In this regard, it will be impossible to start the car engine.
The metal of the oxidized terminals becomes chipped, the contact surface area decreases, which will worsen the conductivity even after they are cleaned and will contribute to the more rapid appearance of oxides during further operation. Therefore, it is necessary to regularly conduct a visual inspection of the battery terminals and take preventive measures related to the elimination of bad contacts and plaque by cleaning them, as well as lubricating the terminals to protect them from oxidation.
If a whitish acid coating appears due to a violation of the integrity of the battery case, then it must be replaced. This is due to the fact that cracks in the battery case from vibrations and shocks during the operation of the car will only increase over time, which will lead to the ingress of acidic electrolyte into the engine compartment with the ensuing disastrous consequences.
The appearance of green plaque on the contacts may indicate high humidity in the parking lot of the car, which can lead to corrosion of its other metal parts.
How to identify signs of terminal oxidation
Oxidation of the battery contacts can be determined both by visual inspection and by indirect signs associated with a decrease in the starting current at the car's starter and a decrease in the brightness of lighting devices.
Visual inspection is associated with the search for microcracks, loosening of the electrodes, traces of electrolyte leakage, the presence of dirt on the ventilation holes. If you are confident that the battery is fully charged, but the engine is cranking slightly with the starter, the cause may be poor contact with the battery.
In order to determine whether the whitish coating is acidic, it is necessary and, using protective gloves, to rinse the contacts and the battery case with a weak solution of soda (up to 10%) in warm water, and then wipe it dry. When an alkaline solution hits the acid, a reaction will occur, accompanied by hissing and heat generation.